Serology
Products
Manual RPR
A nontreponemal test used for the screening of syphilis; a sexually transmitted disease caused by the bacterium Treponema pallidum (a spirochete bacteria).
Details
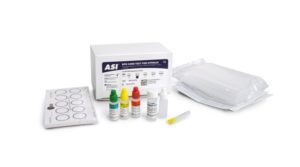 ASI’s nontreponemal RPR (Rapid Plasma Reagin) test screens for syphilis; a sexually transmitted disease caused by the bacterium Treponema pallidum (a spirochete bacteria). The disease is found worldwide and is easily treated if detected early. If left untreated it can be fatal and facilitate the transmission and acquisition of HIV. This test is FDA cleared for use in diagnostic screening.
ASI’s nontreponemal RPR (Rapid Plasma Reagin) test screens for syphilis; a sexually transmitted disease caused by the bacterium Treponema pallidum (a spirochete bacteria). The disease is found worldwide and is easily treated if detected early. If left untreated it can be fatal and facilitate the transmission and acquisition of HIV. This test is FDA cleared for use in diagnostic screening.
Nontreponemal tests, such as the ASI RPR Card Test, are more reliable indicators of untreated syphilis infections and are used to monitor response to treatment or to indicate new infections.
Features
- A CDC recommended primary screening test
- Simple and economical to use
- Easy to interpret
- All testing materials provided
- Available without controls
- 3 levels of liquid controls included (reactive, weak reactive, nonreactive)
- Color-coded dropper vials
- Warp resistant coated test cards
- Screw cap vials (No glass ampules)
- Up to 24 month shelf life (from date of manufacture)
- Proven results (CAP, AAB, API surveys)
- 10, 15, 30 well test cards available
Rubella
A convenient and specific rapid latex particle agglutination test for the qualitative and semiquantitative determination of rubella virus antibodies in serum. Aids in diagnosing recent or active infection and immune status.
Details
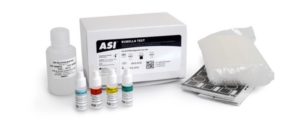 The ASI Rubella test (also known as German Measles) is a convenient and specific rapid latex particle agglutination test for the Qualitative and Semiquantitative determination of rubella virus antibodies in serum. The kit can be used to test diluted or undiluted samples. The test aids in the diagnosis of recent or active rubella infection and in the determination of immune status.
The ASI Rubella test (also known as German Measles) is a convenient and specific rapid latex particle agglutination test for the Qualitative and Semiquantitative determination of rubella virus antibodies in serum. The kit can be used to test diluted or undiluted samples. The test aids in the diagnosis of recent or active rubella infection and in the determination of immune status.
Features
- Excellent reproducibility and outstanding accuracy
- Qualitative and semiquantitative results
- 3 Levels of liquid, ready-to-use controls included
- Results in 8 minutes
- White latex agglutination test
- Correlation with gold standard – Hemagglutination Inhibition Test (HAI)
- Longest shelf life – up to 18 months from date of manufacture
- Made in USA
RF
RF is a chronic, inflammatory autoimmune disorder and a disabling and painful inflammatory condition. This is a specific slide agglutination assay for the qualitative and semiquantitative determination of Rheumatoid Factor (RF) in human serum.
Details
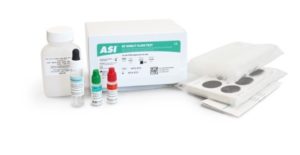 The ASI RF Slide Test is a convenient and specific slide agglutination assay for the Qualitative and Semiquantitative determination of Rheumatoid Factor (RF) in human serum. No initial dilution of patient samples is required for this test.
The ASI RF Slide Test is a convenient and specific slide agglutination assay for the Qualitative and Semiquantitative determination of Rheumatoid Factor (RF) in human serum. No initial dilution of patient samples is required for this test.
Features
- 2 Minute slide test
- Standardized to WHO reference
- No initial dilution required
- 2 Liquid controls included
- Longest shelf life – up to 24 months
- Proudly made in USA
- Proven by CAP, AAB, and API surveys
Automated RPR
A nontreponemal test used for the screening of syphilis; a sexually transmitted disease caused by the bacterium Treponema pallidum (a spirochete bacteria).
Details
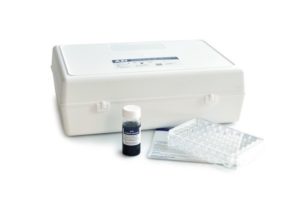 Arlington Scientific’s Automated RPR Syphilis Test is FDA cleared for diagnostic, blood donor screening and cadaveric RPR testing, for use on the ASI Evolution® Automated Syphilis Analyzer. Nontreponemal tests, such as the ASI Automated RPR test, are more reliable indicators of untreated syphilis infections and are used to monitor response to treatment or to indicate new infections. Syphilis, if left untreated, can be fatal.
Arlington Scientific’s Automated RPR Syphilis Test is FDA cleared for diagnostic, blood donor screening and cadaveric RPR testing, for use on the ASI Evolution® Automated Syphilis Analyzer. Nontreponemal tests, such as the ASI Automated RPR test, are more reliable indicators of untreated syphilis infections and are used to monitor response to treatment or to indicate new infections. Syphilis, if left untreated, can be fatal.
Now diagnostic labs, blood banks and tissue banks have an automated RPR syphilis test that uses the CDC’s recommended traditional algorithm for syphilis screening. The ASI Evolution and the Automated RPR test kit offers diagnostic and blood donor screening labs a budget friendly system that will improve workflow efficiencies, reduce labor costs, and provide consistent, dependable, and objective results.
Features
- A CDC recommended primary screening test for Syphilis
- Standardized methodology
- Run 190 tests/hour
- Titers up to 1:2048
- Data management (analyze, archive and retrieve)
- Simple and economical to use
- Reduce labor
- Improve efficiency
- Fifteen minutes to first result
- Made in the USA
Color Mono
Infectious mononucleosis is a contagious disease. Epstein-Barr virus (EBV) is the most common cause of infectious mononucleosis but other viruses can also cause this disease. A color-enhanced test for the detection of heterophil antibodies (associated with IM) in human serum or EDTA plasma.
Details
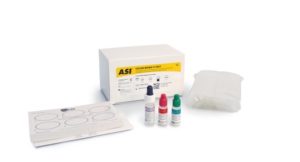 The ASI Color Mono II Test is a simple color-enhanced agglutination test for the qualitative and semiquantitative detection of heterophil antibodies in human serum or EDTA plasma associated with infectious mononucleosis (IM). No initial dilution of patient sample is required for this test. The presence of IM antibodies in serum or plasma at detectable levels will interact with the sensitized particles to produce a visible red agglutination with a blue background, which is a positive result.
The ASI Color Mono II Test is a simple color-enhanced agglutination test for the qualitative and semiquantitative detection of heterophil antibodies in human serum or EDTA plasma associated with infectious mononucleosis (IM). No initial dilution of patient sample is required for this test. The presence of IM antibodies in serum or plasma at detectable levels will interact with the sensitized particles to produce a visible red agglutination with a blue background, which is a positive result.
Features
- Easy to read results in 2 minutes
- Color enhanced agglutination test
- Qualitative and semiquantitative procedures
- All testing materials needed to perform the test included in each kit
- Can be used with serum or plasma
- Color-coded reactive and nonreactive controls with dropper tip vials included in the kit
- Storage requirement: 2-8ºC
- Longest shelf life – up to 24 Months
- Qualitative and semiquantitative procedures
Sickle Cell
A specific test used as an aid in the qualitative detection of hemoglobin S (Hb-S) in anticoagulated whole blood. The test does not distinguish between sickle cell disease (HbS/S) and sickle cell trait (HbS/A).
Details
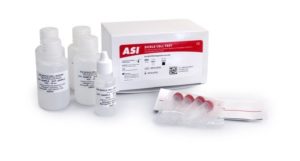 The ASI Sickle Cell Test is a simple and specific test used as an aid in the qualitative detection of hemoglobin S (Hb-S) in anticoagulated whole blood. The test does not distinguish between sickle cell disease (HbS/S) and sickle cell trait (HbS/A). A negative test is normal, a positive test indicates that the person has SCD or sickle cell trait. This test is not recommended for use on newborns under 3 months of age. Kit does not include controls.
The ASI Sickle Cell Test is a simple and specific test used as an aid in the qualitative detection of hemoglobin S (Hb-S) in anticoagulated whole blood. The test does not distinguish between sickle cell disease (HbS/S) and sickle cell trait (HbS/A). A negative test is normal, a positive test indicates that the person has SCD or sickle cell trait. This test is not recommended for use on newborns under 3 months of age. Kit does not include controls.
Features
- Accurate, easy, and economical
- Detects both homozygous (S/S) and heterozygous (A/S) -sickle cell
- Contains urea reagent for test confirmation
- Turbidity line test based upon modified Nalbandian procedure
- Room temperature storage
- Longest shelf life – up to 18 Months from date of manufacture
Staphslide
A convenient and specific slide agglutination assay for the qualitative detection of coagulase (both clumping factor and protein A) to identify Staphylococcus aureus to the exclusion of other species of staphylococci.
Details
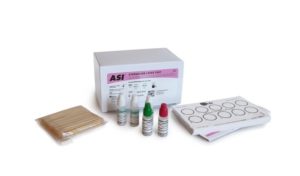 The ASI Staphslide Latex Test is a convenient and specific slide agglutination assay for the qualitative detection of coagulase (both clumping factor and protein A) to identify Staphylococcus aureus to the exclusion of other species of staphylococci. This test is for use on pure culture samples suspected of being S. aureus. The ASI Staphslide Latex Test does detect methicillin resistant S. aureus (MRSA) strains that produce clumping factor and protein A.
The ASI Staphslide Latex Test is a convenient and specific slide agglutination assay for the qualitative detection of coagulase (both clumping factor and protein A) to identify Staphylococcus aureus to the exclusion of other species of staphylococci. This test is for use on pure culture samples suspected of being S. aureus. The ASI Staphslide Latex Test does detect methicillin resistant S. aureus (MRSA) strains that produce clumping factor and protein A.
Features
- Simple to interpret
- 45-Second slide test
- Detects Methicillin Resistant S. aureus (MRSA)
- Liquid controls included
- 100% correlation with tube coagulase test
- Longest shelf life- Up to 12 months
ASO
A specific latex slide agglutination assay for the qualitative and semiquantitative detection of Antistreptolysin-O (ASO) antibodies in human serum. No initial dilution of patient sample is required for this test.
Details
 The ASI ASO Latex Slide Test is a convenient and specific latex slide agglutination assay for the qualitative and semiquantitative detection of Antistreptolysin-O (ASO) antibodies in human serum. No initial dilution of patient sample is required for this test.
The ASI ASO Latex Slide Test is a convenient and specific latex slide agglutination assay for the qualitative and semiquantitative detection of Antistreptolysin-O (ASO) antibodies in human serum. No initial dilution of patient sample is required for this test.
Features
- 2 Minute slide test
- 2 Liquid controls included
- Longest shelf life – up to 18 month
- Proven by AAB, CAP, and API surveys
VDRL
A nontreponemal test used for the screening of syphilis. This VDRL test can also be used to detect if syphilis has involved the central nervous system (Neurosyphilis) by testing cerebral spinal fluid.
Details
 The ASI VDRL Antigen Test is a nontreponemal test used for the screening of syphilis. VDRL is a specific qualitative (Positive or Negative) and semi-quantitative test (concentration of) that detects reagin antibodies (proteins), in human serum. This VDRL test can also be used to detect if syphilis has involved the central nervous system (Neurosyphilis) by testing cerebral spinal fluid.
The ASI VDRL Antigen Test is a nontreponemal test used for the screening of syphilis. VDRL is a specific qualitative (Positive or Negative) and semi-quantitative test (concentration of) that detects reagin antibodies (proteins), in human serum. This VDRL test can also be used to detect if syphilis has involved the central nervous system (Neurosyphilis) by testing cerebral spinal fluid.
Kits include two vials of VDRL antigen (2 x 5mL), two vials of VDRL buffered saline (2 x 60mL). The kit could perform as many as 5,900 tests but it is generally less due to facility testing workflow.
Features
- Suitable for testing cerebral spinal fluid (CSF) specimens
- Meets CDC primary algorithm recommendation
- Easy to interpret
- Simple and economical to use
- Screw cap vials – No glass ampules
- Room temperature storage
- Long shelf life – up to 60 months from date of manufacture
- Results on CAP, AAB and API surveys
- Made in USA
Color Staph
A rapid, color enhanced latex agglutination test to detect clumping factor and/or Protein-A, characteristics associated with Staphylococcus aureus colonies obtained from culture. Color enhanced for visual reading.
Details
 The ASI Color Staph is a rapid, color enhanced latex agglutination test to detect clumping factor and/or Protein-A, two characteristics associated with Staphylococcus aureus colonies obtained from culture. ASI Color Staph latex reagent will react with either or both of these characteristics. The aggregation of the smooth latex suspension represents a positive reaction which is visible to the unaided eye producing a red agglutination in a “blue” background.
The ASI Color Staph is a rapid, color enhanced latex agglutination test to detect clumping factor and/or Protein-A, two characteristics associated with Staphylococcus aureus colonies obtained from culture. ASI Color Staph latex reagent will react with either or both of these characteristics. The aggregation of the smooth latex suspension represents a positive reaction which is visible to the unaided eye producing a red agglutination in a “blue” background.
Features
- Simple to interpret
- 10-30 Second slide test
- Detects Methicillin Resistant S. aureus (MRSA)
- Liquid controls included
- Longest shelf life- Up to 24 months
- Made in USA
CRP
C-Reactive Protein (CRP) is a protein produced by the liver in response to acute injury, infection, or inflammation. Detects C-Reactive Protein (CRP) in human serum. No initial dilution of patient sample is required for this test.
Details
 The ASI CRP Slide Test is a convenient and specific slide agglutination assay for the qualitative and semiquantitative detection of C-Reactive Protein (CRP) in human serum. No initial dilution of patient sample is required for this test.
The ASI CRP Slide Test is a convenient and specific slide agglutination assay for the qualitative and semiquantitative detection of C-Reactive Protein (CRP) in human serum. No initial dilution of patient sample is required for this test.
Features
- 2 Minute slide test
- No initial dilution required
- 2 Liquid controls included
- Longest dating – up to 24 month shelf life
- Proven by AAB, CAP and API surveys
For information about our entire line of Serology please send us an email by clicking the link below:


